Abstract
Introduction: Recombinant factor IX Fc fusion protein (rFIXFc) is an extended half-life therapy for severe hemophilia B. The safety, efficacy, and prolonged half-life of rFIXFc were demonstrated in previously treated pediatric, adolescent, and adult subjects with severe hemophilia B in the Phase 3 B-LONG and Kids B-LONG trials (NCT01027364 and NCT01440946, respectively) (Fischer et al, Lancet Haematol, 2017; Powell et al, N Engl J Med, 2013). Here, the final results are reported from B-YOND (NCT01425723), the long-term extension of those 2 studies.
Methods: This was an open-label, multicenter, long-term trial of previously treated subjects of all ages with severe hemophilia B. Dosing regimens included weekly prophylaxis (WP; 20-100 IU/kg every 7 days), individualized prophylaxis (IP; 100 IU/kg every 8-16 days or twice monthly), modified prophylaxis (MP; for subjects not achieving optimal dosing on IP or WP), or episodic treatment (ET; on-demand dosing based on type and severity of bleeding episodes). Subjects <12 years of age could only enroll in the prophylactic groups (WP, IP, or MP). Investigators were permitted to change treatment groups for a subject at any point in B-YOND; thus, subjects may be included in >1 group for analyses. The primary endpoint was development of inhibitors. Other endpoints included annualized bleeding rates (ABRs), joint ABRs, spontaneous joint ABRs, exposure days (ED), and factor consumption. Descriptive statistics were used for analysis. Analyses were performed separately based on parent study.
Results: A total of 120 subjects (93 from B-LONG and 27 from Kids B-LONG) enrolled in B-YOND, and 98 subjects (75 from B-LONG and 23 from Kids B-LONG) completed the study. Of the 93 subjects from B-LONG, the median (range) age was 29 (13‒63) years and most were prescribed WP (WP, n=51; IP, n=31; MP, n=17; ET, n=15). Subjects from Kids B-LONG had a median (range) age of 7 (3‒12) years. Among subjects <6 years of age, 13 were prescribed WP and 1 MP. Ten subjects 6 to <12 years of age received WP, 5 received IP, and 1 received MP.
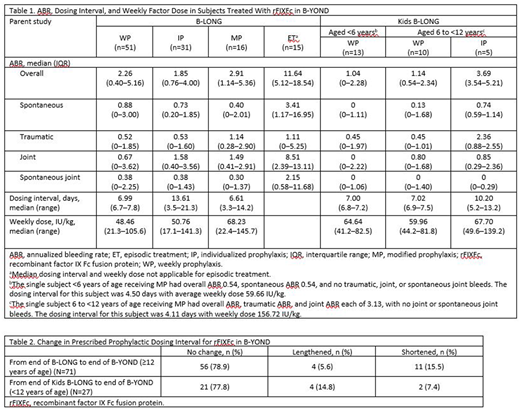
No subject developed an inhibitor during B-YOND. The overall safety profile of rFIXFc was consistent with the parent studies and prior interim analyses. ABRs remained low and stable throughout B-YOND (Table 1). The overall median (range) number of ED in B-YOND for subjects from B-LONG was 146.0 (8.0-462.0) days, with an overall median (range) duration of 208 (4 years; 13.9-280.0) weeks. For subjects from the Kids B-LONG study, the median (range) number of ED was 55.0 (8.0-204.0) days and 149.0 (53.0-202.0) days for those aged <6 years and 6 to <12 years, respectively. Median (range) exposure to rFIXFc was 55.0 (1.1 years; 7.9‒177.0) weeks for subjects <6 years of age and 175.66 (3.4 years; 47.0‒201.1) weeks for subjects 6 to <12 years of age.
The median dosing interval ranged from 7 to 14 days for B-LONG subjects and 7 to 10 days for Kids B-LONG subjects (Table 1). Most subjects (79% and 78% from B-LONG and Kids B-LONG, respectively) had maintained their dosing interval during the extension trial (Table 2), although the dosing interval was lengthened for 6% of B-LONG subjects and 15% of Kids B-LONG subjects. Median weekly dose was low (Table 1) and ranged from 48.46 IU/kg to 68.23 IU/kg for B-LONG subjects and 59.96 IU/kg to 67.70 IU/kg for Kids B-LONG subjects. There was no change in median weekly factor consumption from the end of either parent study through the B-YOND extension.
Conclusions: During up to 4 years of treatment with rFIXFc in the B-YOND study, no inhibitors were reported. The low ABRs, low consumption, and extended dosing intervals of up to 14 days that were observed during the parent studies were sustained for the duration of the long-term extension trial. These data confirm the consistent, well-characterized safety profile, and durable efficacy of rFIXFc prophylaxis in subjects of all ages with severe hemophilia B.
Ragni:Alnylam: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; American Academy of CME: Honoraria; Baxalta/ Shire: Research Funding; Baxter Bioscience: Research Funding; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Biogen/Bioverativ: Consultancy, Research Funding; Biomarin: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; CSL Behring: Research Funding; Foundation for Women and Girls with Blood Disorders: Membership on an entity's Board of Directors or advisory committees; Institute for Cost Effectiveness Research (ICER): Consultancy; NovoNordisk: Research Funding; OPKO Biologics: Research Funding; Pfizer: Research Funding; Shire Development LL: Consultancy, Other: Non-financial support (study Drug); SPARK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; MOGAM(Green Cross Corporation: Membership on an entity's Board of Directors or advisory committees. Kulkarni:Bioverativ: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; NovoNordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Shire: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Octa Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kedrion: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genetech: Honoraria, Membership on an entity's Board of Directors or advisory committees; BPL: Honoraria, Membership on an entity's Board of Directors or advisory committees. Pasi:Biomarin: Honoraria, Research Funding; NovoNordisk: Speakers Bureau; Alnylam: Honoraria, Research Funding; Catalyst Bio: Honoraria; Shire: Speakers Bureau; Octapharma: Honoraria; Pfizer: Speakers Bureau; Apcintex: Honoraria; Bioverativ: Honoraria, Research Funding; Sobi: Honoraria; Bayer: Speakers Bureau. Mahlangu:Sanofi: Research Funding, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau; LFB: Consultancy; NovoNordisk: Consultancy, Research Funding, Speakers Bureau; CSL Behring: Consultancy, Research Funding, Speakers Bureau; Chugai: Consultancy; Catalyst Biosciences: Consultancy, Research Funding; Biomarin: Research Funding, Speakers Bureau; Biogen: Research Funding, Speakers Bureau; Bayer: Research Funding; Amgen: Consultancy; Alnylam: Consultancy, Research Funding, Speakers Bureau; Shire: Consultancy, Research Funding, Speakers Bureau; Sobi: Research Funding, Speakers Bureau; Spark: Consultancy, Research Funding. Shapiro:Bioverativ, a Sanofi Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Research Funding; BioMarin: Research Funding; Bayer Healthcare: Other: International Network of Pediatric Hemophilia; Shire: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Prometic Life Sciences: Consultancy, Research Funding; Octapharma: Research Funding; Kedrion Biopharma: Consultancy, Research Funding; Sangamo Biosciences: Consultancy; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding; OPKO: Research Funding; Bio Products Laboratory: Consultancy; Genetech: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Nolan:Bayer: Research Funding; CSL Behring: Research Funding; Sobi: Research Funding. Oldenburg:Chugai: Honoraria, Membership on an entity's Board of Directors or advisory committees; Grifols: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Biogen Idec: Honoraria, Membership on an entity's Board of Directors or advisory committees; Shire: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Octapharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Biotest: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Swedish Orphan Biovitrum: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Matsushita:Bioverativ: Honoraria; Bayer: Honoraria, Research Funding; NovoNordisk: Honoraria, Research Funding; JB: Honoraria, Research Funding; Shire: Honoraria; CSL: Honoraria. Willemze:SOBI: Employment. Rudin:Bioverativ: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal